Types of Ionizing Radiation
Posted August 20, 2016 by David Schumann

If we remember that all matter is composed of atoms, and atoms are composed of protons, neutrons, and electrons, then we have the basis to understand ionizing radiation. For what we are concerned with, each atom typically has an electrical balance of protons, neutrons, and electrons. Protons have positive charge, neutrons have no charge (they’re neutral), and electrons have negative charge. The nucleus of the atom is comprised of protons and neutrons, and the electrons whirl around the atom in various and discrete energy levels.
Most atoms are stable, but we are concerned about the type of atoms that are unstable. They are unstable because they have an excess of energy, mass, or both, and this instability means they give off excess energy or mass. Unstable atoms are said to be radioactive because these emissions of energy or mass are called radiation.
Of the ways in which an atom can emit radiation, there are four main ways they can shed this excess energy. Alpha and beta radiation, along with neutrons (considered indirectly ionizing), are emissions of energy and mass in the form of particles. Gamma rays and X-rays are emissions of energy in the form of electromagnetic waves and massless particles called photons.
Alpha particles
The nucleus of a helium atom consists of two neutrons and two protons, the same as an alpha particle. These emissions have a high mass and travel short distances. While heavy and damaging, they can’t penetrate through a piece of paper or our skin. Inside the body they can and do cause heavy damage in our tissues because of their mass and momentum. They are considered high LET (linear energy transfer) radiation and are released by the most deadly radioactive isotopes such as plutonium, polonium, uranium, and radium.
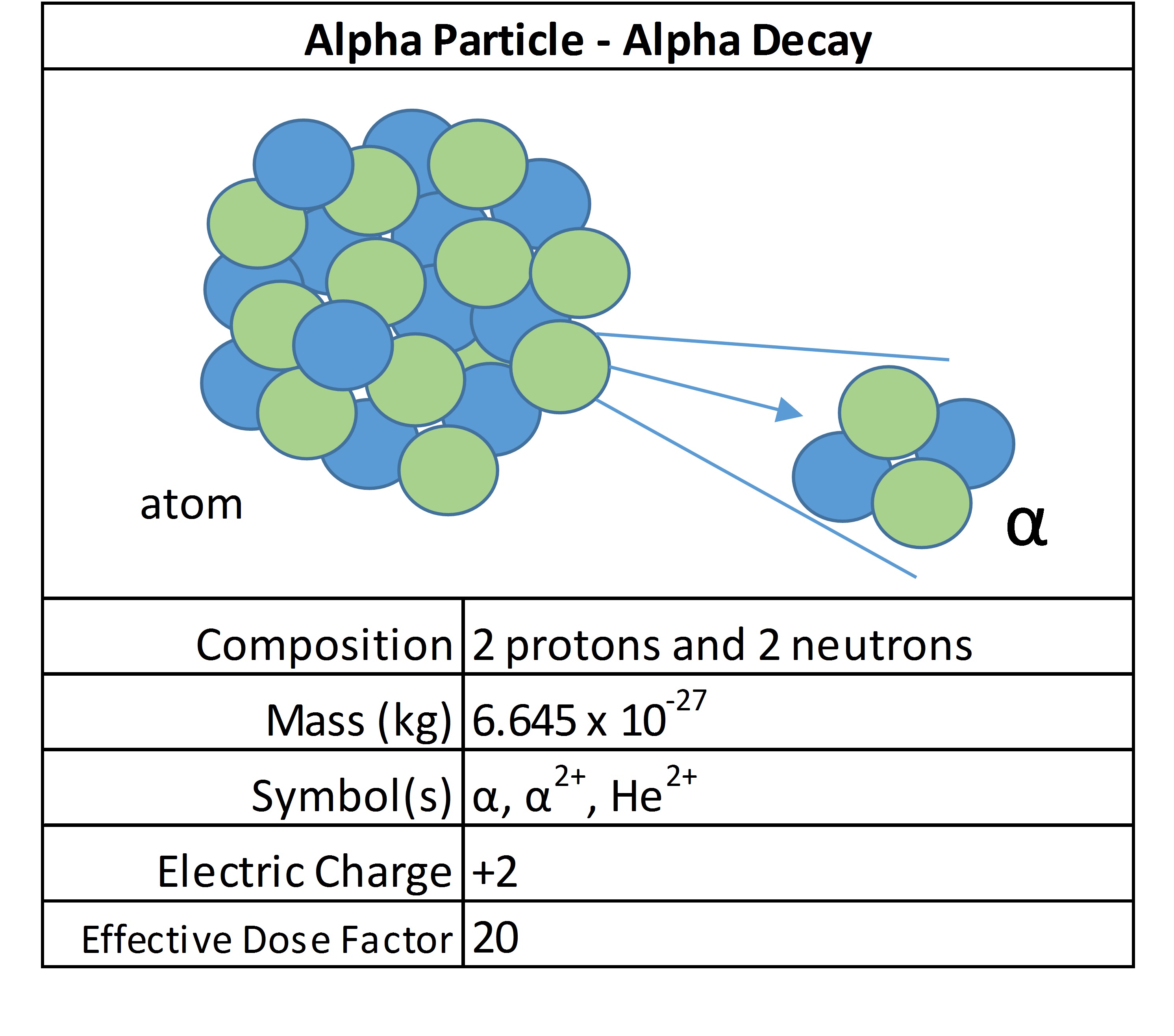
Beta particles
Beta emissions consist of electrons or positrons. They have a small mass and have variable energy. They can be stopped by a thick piece of plastic. They are considered low LET radiation and most radioactive isotopes release beta radiation.
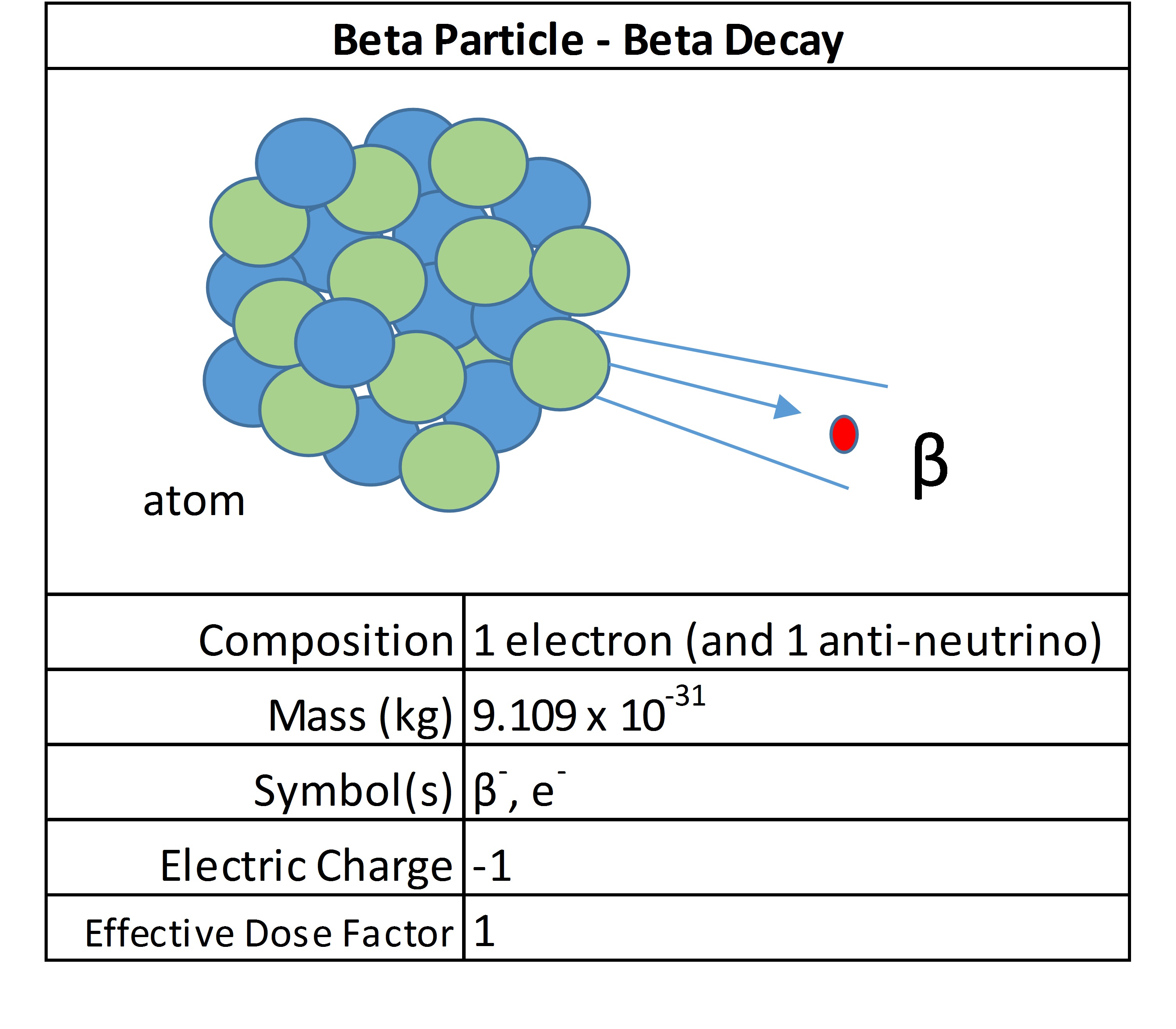
Gamma Rays / X-Rays
Gamma rays and X-rays, comprised of massless photons, are released when the nucleus of an atom releases energy, typically after an alpha, beta or positron transition. They are waves of high energy and penetrating radiation. There is some overlap, but in general, gamma rays have higher energies and frequencies compared to X-rays.
They both stem from the same phenomenon: radiation emissions are released from atoms when inner-shell electrons are removed. The remaining electrons rearrange and in balancing the energy as a high energy ray. Higher density materials are required to stop this form of ionizing radiation. Commonly, a plate of lead is used to block them. They are considered low LET radiation and produce fast electrons in the body that cause damage.
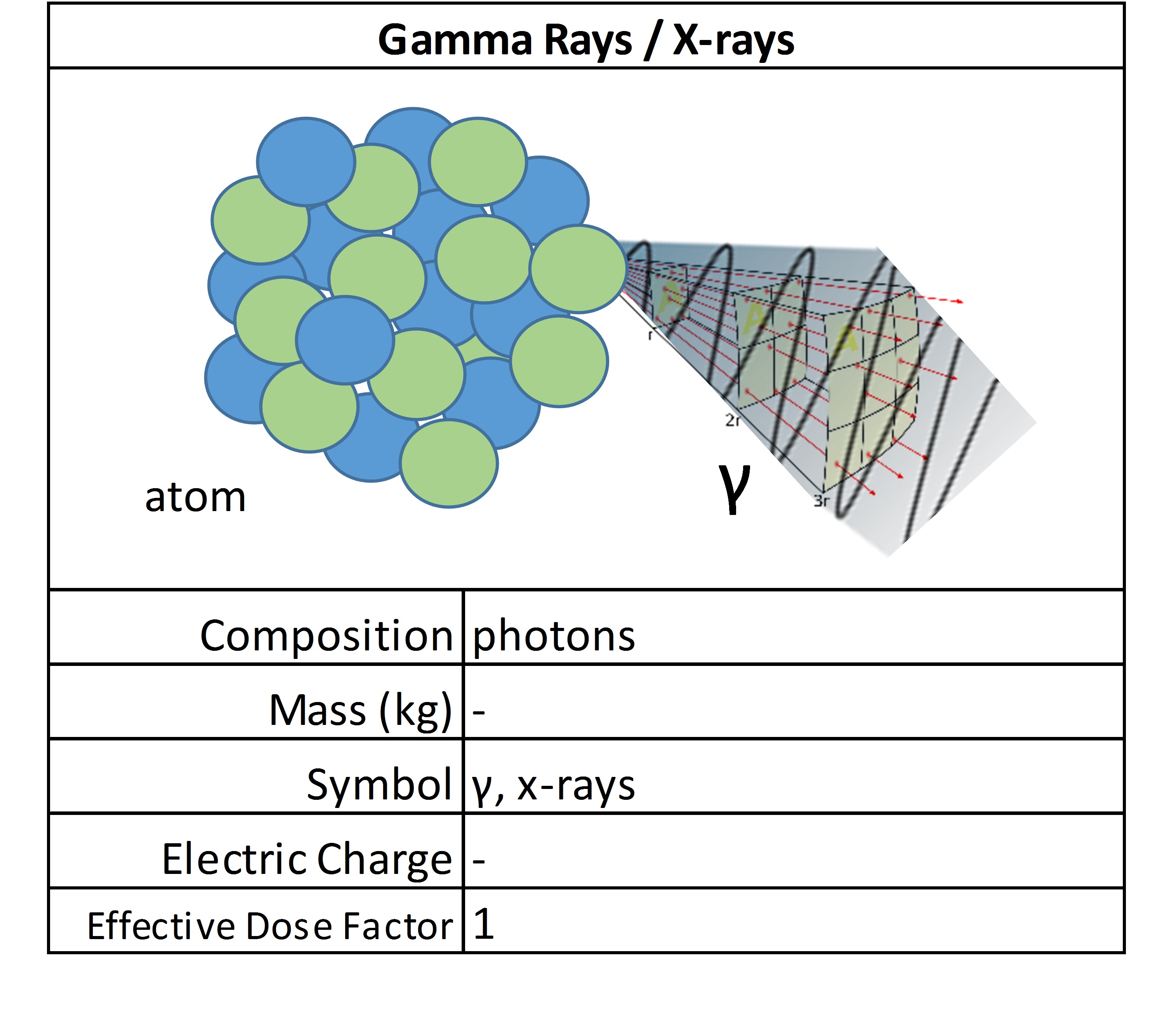
Neutrons
These uncharged particles don’t directly cause ionization, but they are considered indirectly ionizing. Because they have no charge, they are a very penetrating form of radiation. They have enough speed to strike other atoms, usually hydrogen, which then causes the impacted atom to give off a form of primary ionizing radiation, such as a beta emission.
Depending on their source, their strength and dose calculation factors can vary. There are free neutrons everywhere in space, and yet they are released abundantly during fission reactions. Neutrons are excellent at starting nuclear reactions because they are not repulsed by other charged particles and can cause certain radioactive materials to split or fission. It is the chain reaction of neutron releases that sustains nuclear reactions. They are considered medium LET radiation because while having no charge, they have a high mass.
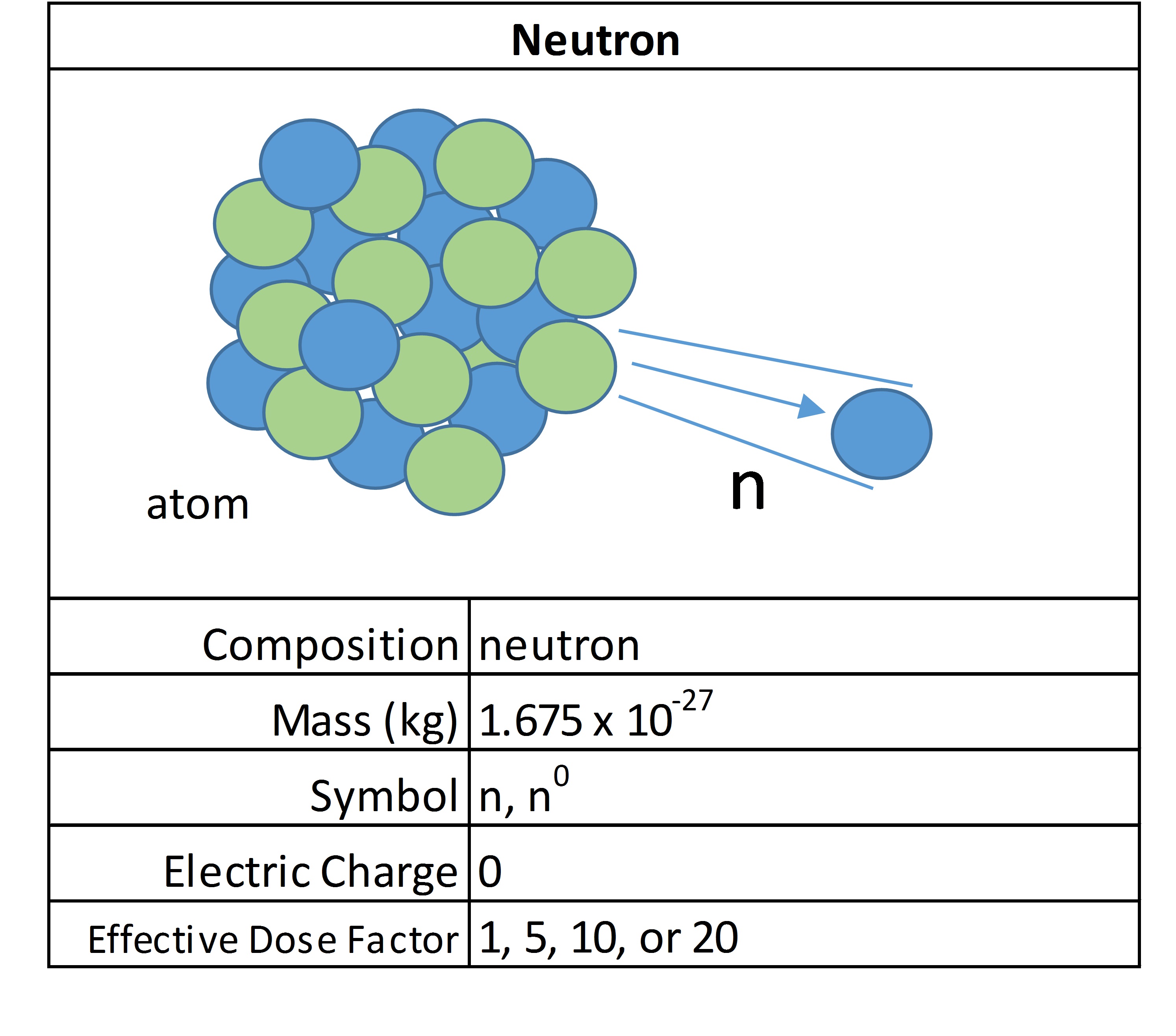
Penetrating Ability
Alpha particles are extremely damaging, but easy to stop and shield. This means externally they pose no threat, but internally can cause serious damage. Where medical professionals use x-rays, they typically use lead shielding to prevent exposure to unintended areas. Neutrons are very difficult to stop because they have no charge and are not repulsed by atoms, which is why nuclear weapons engineers and scientists created the neutron bomb.
Early nuclear weapons were focused on releasing large amounts of destructive energy. Because an enemy can bunker and hide from this and it causes destruction to structures in an area, bomb-designers wanted a new type of bomb that would kill lots of people, but with less damage. A neutron bomb is called an enhanced radiation weapon because it promotes massive quantities of high energy neutrons to be released, which pass through most shielding to inflict the maximum amount of casualties. It is a terrible creation and a reminder that we desperately need to keep focus on nuclear non-proliferation and warhead dismantlement.
Radiation Measurement
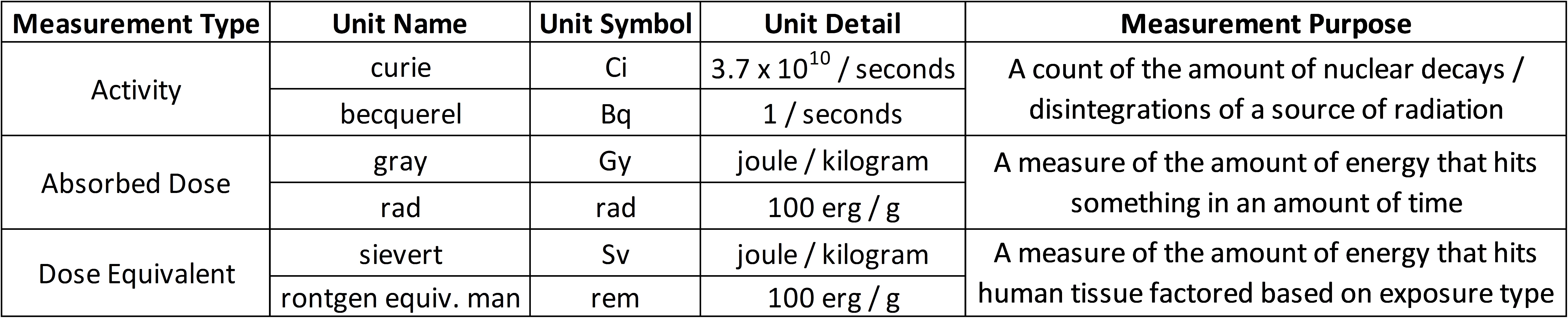
Accounting for radiation needs various units of measure. The International System of Units (SI units) measurements are most widely accepted, but in the United States, older measurements persist. There are three primary types of measurements we are concerned with:
Becquerel (Bq) — Same as the Curie, it is a measure of the amount of decays, or disintegrations, per second, of a particular source of radiation. It measures the activity of some source of radiation.
Gray (Gy) — A measure of the absorbed energy into an amount of matter, or the total amount of energy that hits something in an amount of time. The units are energy divided by mass (joules per kilogram) of matter (same as rads). It measures the absorbed dose of radiation into some mass of matter.
Sievert (Sv) — A measure of the absorbed energy into an amount of biological matter, expressed in joules per kilogram of living tissue. The Sievert, or rem, is a calculated value that equals the absorbed dose multiplied by a quality factor that modifies the dose value so that it correlates with health effects based on studies. These factors take into account the nuances of radiation on biological tissue.
For additional information about ionizing radiation and what you can do to protect yourself and your loved ones from its harmful effects, purchase a copy of Living With Radiation. Heal and Protect: A Nuclear World.
Comments ( 0 )