Radiation and the Dangers of Ionization
Posted February 18, 2017 by David Schumann
Radiation is nothing more than energy that propagates through space. This energy is comprised of energetic particles and electromagnetic waves that interact with the environment and our bodies. There are many sources, but atoms that release energy go through a process called ionization, which creates ionizing radiation – the type of radiation we are concerned with here.
Ionization is a unique and natural phenomenon that we are surrounded by and have learned to exploit, creating absolutely wondrous and horrifically unnatural effects. Because small amounts of matter contain very large amounts of energy, what we can do with nuclear technology is amazing. We can destroy our world in the blink of an eye, drive massive ships around the sea for decades, power cities and nations, peer into the body, and save lives. It is no wonder humankind is so enthralled by the power of nuclear forces. The atom is the definition of high energy density, and the atom is the source of ionizing radiation.
Ionizing radiation is named so because it has enough energy to dislodge electrons from their atomic orbits, thereby ionizing the atom. An ion is an atom where the number of protons are not equal to the number of electrons, giving the atom a net positive or negative charge. Instead of passing through us somewhat harmlessly, as most non-ionizing radiation does, ionizing radiation tends to crash into us and more significantly interact with our tissues and cells, and the atoms that comprise them. This interaction causes tissue damage and a host of health issues. Ionizing radiation includes energetic particles, such as alpha and beta radiation, and electromagnetic waves, such as x-rays and gamma rays, which are part of a broader spectrum of electro-magnetic (EM) radiation.
Technology and science in the last century and a half has been about utilizing this wide spectrum of electromagnetic radiation for many purposes. On one end of the EM spectrum are radio and television waves, with longer wavelengths and lower frequencies. Microwave radiation in the gigahertz frequency-range is responsible for mobile/cellular communications and heating of food. Non-ionizing extends above these frequencies into infrared and visible light.
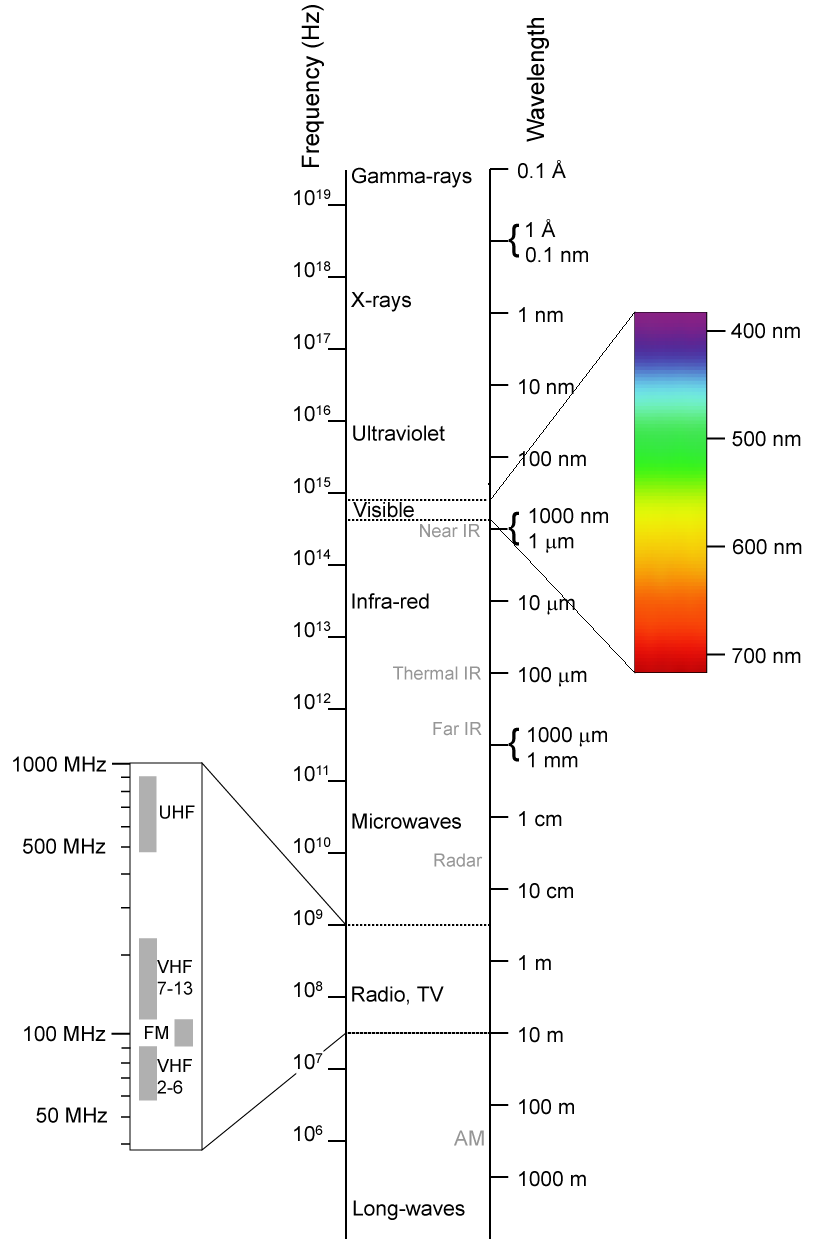
The Electromagnetic (EM) Spectrum
Because of the longer wavelengths and lower frequencies, radiation that does not cause ionization tends to pass through most things with less effects, hence the reason why radio and television transmissions can travel far and reach into our homes. This property begins to end with visible and ultra-violet light which reflects off of matter more readily. The high ultraviolet (UV) frequencies are where non-ionizing radiation becomes ionizing radiation. For the most part, non-ionizing radiation does not present a serious health threat to humans because the longer wavelengths interact less with our bodies. Non-ionizing radiations (like cell-phones) do have effects on the body: vibrating, heating, and interacting with our cells and tissues. Don’t assume this radiation is benign without any health effects. Please see Heal and Protect: A Wireless World.
Electromagnetic radiation that causes ionization, such as gamma rays and x-rays, has very short wavelengths and extremely high frequencies. This radiation has more effects and collides or interacts significantly with matter. This is a pro and a con. On one side we can stop the penetration easily, but on the other side, this radiation damages us. This is the radiation we are mostly concerned about because it has significant effects on our bodies.
Ionizing radiation starts in the ultra-violet light frequencies, created by the sun. The UV exposure we receive from the sun, however, generally does not cause ionization because the atmosphere protects us. It lacks the energy to ionize an atom, but it does cause chemical changes and interacts with organic molecules. This causes us to suntan, freckle, and allow our bodies to synthesize vitamin-D, but also can damage tissues and lead to skin cancers.
Radioactivity is the spontaneous release of energy from an atom. Radioactive elements release radiation because they are unstable atoms. These unstable atoms are called radionuclides and they don’t differ much from stable atoms except they continually shed excess energy and mass, in the form of ionizing radiation. The types of radiation that cause ionization can be particle radiation (alpha/beta/neutron), EM radiation (gamma/x-ray), or a combination.
Radioactive elements are used to create nuclear energy and weapons, which in turn, release tremendous amounts of radiation and energy. Ionizing radiation has negative effects on biological systems with increased cumulative exposure to it resulting in increased risk of mutation, disease, cancer, or death. Ionizing radiation can cause breaks in DNA strands leading to mutations, and can contribute to free-radical pathology and other negative effects in the body. Atomic energy, whether released quickly in the flash of a bomb, or slowly in the steady decay of radioactive elements, is damaging and dangerous — always — to life as we know it.
Properties of Radiation & Ionization
The world has been caught in the allure of the immense power and interesting properties of nuclear forces and radioactive substances for a long time.
The first radioactive element to find its way into popular imagination was Radium – the Giver of Rays. It was discovered by Marie and Pierre Curie at the end of the 19th century. Its unique properties bewitched scientists, doctors, and lay-people for the next 70-80 years and many unnecessary cancers and health issues were the result of the use of radium for just about everything, including removing skin blemishes, treating gynecological issues, and even making virility creams, toothpastes, and health tonics. Pierre would carry around vials of radioactive radium in his vest and show people how it glowed in the dark.
Glowing and illumination were some of the earliest properties used from radioactive elements and they are still used for this purpose today. Radium is banned from consumer products and has been replaced by safer radioactive isotopes.
Pierre and Marie Curie both suffered greatly and died early because of their work with radiation in the early days of this new science. Even though many suspected issues, it still took a long time for the laboratories of the world to fully respect how serious those hazards were. Most of the early pioneers of nuclear science succumbed to radiation sickness, cancers and death from their profession.
Medically, radiation is used to save lives through imaging techniques, such as X-Ray and CT/PET-scan, and through the application of radiation to kill cancerous tumors. It is ironic that radiation both kills and is used medically to extend life and improve diagnostics.
The discovery and development of X-Rays and the realization they were harmful to health is a fascinating story, but it is through this process that the early scientists first attempted to develop techniques to measure radiation. The benefits of X-rays were clear, but the amount of energy being released by early X-ray tubes was not.
The property that has caught humanities attention, however, is the ability to exploit the incredible amounts of energy in the atom. Radioactive elements are the key to harnessing and deploying this terrible and tremendous energy contained within the smallest amount of matter. Nuclear power plants and nuclear bombs are the result of decades of science and research, but they owe their possibility to the naturally occurring radioactive element of uranium.
Uranium is the most important radioactive element and it occurs naturally. It was discovered in the late 18th century by the German chemist Martin Klaproth, but didn’t find a real purpose until the 20th century where it changed the world forever. Named after the plant Uranus, it is interesting that astrologically, Uranus is the planet that brings disruptive changes in unpredictable ways. One side of Uranus is idealistic genius and the other side is impulsive irresponsibility. These two sides describe the creation and implementation of nuclear energy and weapons perfectly.
The possibility of nuclear energy was realized by scientists at the turn of the 20th century, when they learned, through Einstein’s equations, the incredible amount of energy small amounts of mass contain. The equations showed the possibility of unleashing incredible energy, but this door was closed. Uranium was the key that opened that door.
Uranium is everywhere in the Earth’s soil and water. Formed in stars and blasted across the universe in supernovae, the uranium here is older than the Earth. There are two naturally occurring isotopes, uranium-235 and uranium-238.
U-235 is the valued isotope because it will engage in and sustain fission reactions that release the tremendous energy in the atom. Less than 1% of naturally occurring uranium is U-235, which is why nuclear energy and weapons start with a carefully guarded process of enrichment to create higher concentrations of it. After enrichment, the depleted uranium (depleted of U-235 and nearly 100% U-238) is used further in shielding and armor piercing weapons where the high density and unique properties of Uranium make it valued.
Humankind’s exploitation of the nuclear forces in the atom is one of our greatest technological achievements, but one with great and terrible consequences to be managed. The unique properties of radioactive materials and the unparalleled energy density they open for us have bestowed electrical power, political power, healing power, and world-destroying power. They have also left a legacy of pollution and global contamination that threatens the health of our species.
Comments ( 0 )